Class 10 Science
Chemical Reactions and Equations


Question1.
Magnesium ribbon burns with a dazzling white flame and changes into a white powder. This white power is
(a) Magnesium trioxide
(b) Magnesium chloride
(c)Magnesium hydroxide
(d)Magnesium oxide
Question2.
Decomposition of vegetable is an example of-
(a) Exothermic process.
(b) Endothermic process.
(c) Combination process.
(d) Displacement reaction.
Question3.
Balance the Following reaction-
i. SbCl3 + LiAlH4 à SbH3 + LiCl + AlCl3
ii. NH4Cl + Ca(OH)2 à NH3 + CaCl2 + H2O
Question4.
When Iron salt is treated with a strong alkali it produces a reddish ppt. Write down the reaction and name of the compound?
Question5.
What if Quick lime absorbs the atmospheric moisture and becomes
(a)white powder, slaked lime and exothermic process
(b) yellow solution , carbonate solution and endothermic process
(c) white crystals, CaCl2 and exothermic process
(d) Stay as it is , no heat will evolve.
Question6.
Translate the following statements into chemical equations and then balance them.
(a) Sodium carbonate is reacted with Hydrochloric acid it gives sodium salt with water , carbon dioxide is released during the reaction.
(b) When Magnesium chloride is reacted with ammonium hydroxide it gives magnesium hydroxide and Ammonium chloride.
Question7.
Define combination reaction. Explain a combination reaction with the help of water and oxygen.
Question8.
Balancing chemical reaction follows
(a) Law of conservation of mass.
(b) Law of constant proportion.
(c) Law of multiple proportions.
(d) Law of reciprocal proportion or law of equivalent proportion.
Question9.
Boy mixed an acid in a water filled test tube, the teacher told him not to do it while he mixed it. What will he observe then?
Question10.
What do you understand by skeleton reaction or equation?
Question11.
What is decomposition reaction give some example of
(a) thermal decomposition.
(b) photo decomposition.
Question12.
Black coating on silver and green coating on Copper is an example of –
(a) Corrosion.
(b) Rancidity.
(c) Reduction.
(d) Deposition.
Question13.
Your friend came back after a long time and bring some homemade snacks for you, He keep it somewhere and forget it after some month he senses bad smell in air, what it be?
Question14.
What happen to iron rod if it is–
(a) Immersed in water for a long time.
(b) Keep it in an open environment.
(c) Immersed in oil.
(d) Dipped in NaCl solution.
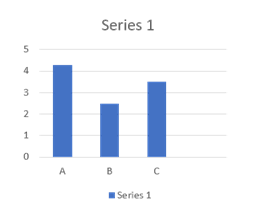
Question15.
According to the given graph,
A,B & C are three iron nails (corrode iron, pure iron and coated iron) reacted with copper sulphate solution, given data shows the reactivity of iron with copper shows that –
(a) Highest reactivity of iron nail.
(b) Strength of iron nail.
Question16.
Formation of ozone is an example of-
(a) Decomposition reaction
(b) Combination reaction
(c) Exothermic reaction
(d) Endothermic reaction
Question17.
What is chemical reaction and how would you determine that chemical reaction took place?
Question18.
What happens when lead nitrate is heated?
(a) Lead oxide is formed.
(b) Lead nitrate will not break.
(c) Product dissolves in the solution
(d) Pure lead is formed and nitrogen gas is released
Question19.
Define double displacement reaction and precipitation reaction by using only examples for both.
Question20.
Fill in the blanks and identify the type of chemical reaction.
(a) PbNO3 + Na2SO4 à_____ + NaNO3
(b) 2Al + Fe2O à ____+ 2Fe
(c) C6H12O6 à 6CO2 + ____
(d) MgO + H2O à _____
**********
In summary, problem-solving after learning a theoretical concept on CBSE Chemical Reactions and Equations Class 10 Science is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 10 Science Chemical Reactions and Equations NCERT Chapter 1
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 10 Science Chemical Reactions and Equations NCERT Chapter 1 useful.
Last but not least, to get the best hold on Class 10 Science Chemical Reactions and Equations NCERT Chapter 1, do not forget to check out: