Class 11 Chemistry
Chemical Bonding and Molecular Structure


Question1.
Number of π bonds and σ bonds in the following structure is
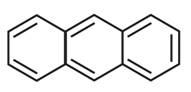
1) 7, 23
2) 7, 25
3) 7, 28
4) 7, 26
Question2.
If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2 5s2, the four electrons involved in chemical bond formation will be_____.
(i) 4p6
(ii) 4p6, 5s2
(iii) 4p6, 4d2
(iv) 4d2, 5s2
Question3.
Which of the following angle corresponds to sp hybridisation?
(i) 90°
(ii) 120°
(iii) 180°
(iv) 109°
Question4.
The molecular formula of the compound formed from B and Cl will be
(i) BCl
(ii) B2Cl
(iii) BCl2
(iv) BCl3
Question5.
Which among the following is paramagnetic?
(i) C2
(ii) N22–
(iii) F2
(iv) O22–
Question6.
1) if Nb is greater than Na
2) if Bond order is positive
3) if Na is less than Nb
4) if Bond order is negative
Question7.
Explain whether melting point of compound showing intermolecular H-bonding is more or less than melting point of compound showing intramolecular H-bonding.
Question8.
Draw the resonance structures of Ozone molecule.
Question9.
NO+ is isoelectronic with
(i) CO
(ii) N2
(iii) PbCl2
(iv) NO2–
Question10.
Define
a) Bond Length
b) Bond angle
c) Bond Enthalpy
Question11.
State main postulates of VSEPR theory.
Question12.
Match the following:
Molecules Geometry
1) BeCl2 a) Tetrahedral
2) CH4 b) Octahedral
3) SF6 c) Trigonal bipyramidal
4) BF3 d) Linear
5) PCl5 e) Trigonal planar
Question13.
Match the following:
Column 1 Column 2
1) Hydrogen bond a) sp3d
2) Polar Covalent Bond b) HF
3) SF4 c) HCl
4) BrF5 d) Linear
5) HC ≡ CH e) sp3d2
Question14.
The electronic configuration of the outer most shell of the least electronegative element is
(i) 2s2 2p5
(ii) 3s2 3p5
(iii) 4s2 4p5
(iv) 5s2 5p5
Question15.
In HNO3, the number of bond pairs and lone pairs of electrons on nitrogen atom are
(i) 2, 2
(ii) 3, 1
(iii) 1, 3
(iv) 4, 0
Question16.
In CO32– ion the formal charge on the oxygen atom of C–O bond is
(i) + 1
(ii) – 1
(iii) – 0.75
(iv) + 0.75
Question17.
What is octet rule? State limitations of Octet Rule.
Question18.
Define Lattice Enthalpy with an example.
Question19.
Which of the following species have identical bond order?
F2, NO+, CN-, O22-
Question20.
Define Dipole moment. State its unit.
**********
In summary, problem-solving after learning a theoretical concept on CBSE Chemical Bonding and Molecular Structure Class 11 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Chapter 4
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Chapter 4 useful.
Last but not least, to get the best hold on Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Chapter 4, do not forget to check out: