NEET Chemistry
Structure of Atom


Question 1.
If Rutherford had used gamma rays (γ-rays) instead of alpha particles (α-particles) in his gold foil experiment, which of the following observations would not have been made?
[Level: Easy]
(a) Gamma rays passing through the foil without any deflection.
(b) Gamma rays being significantly deflected by the nucleus of the atoms.
(c) Very few gamma rays getting absorbed by the foil.
(d) The scattering of gamma rays by the electrons in the gold atoms.
Question 2.
A 500-W radio transmitter operates at a frequency of 950 Hz. How many quanta per second does it emit?
[Level: Difficult]
(a) 8.03×1021
(b) 7.03×1029
(c) 5.93×1030
(d) 7.95×1029
Question 3.
An electron is confined to move freely inside a one-dimensional box of length 5 cm. What is the minimum uncertainty in its velocity?
[Level: Moderate]
(a) 2×10-3 m/s
(b) 1×10-3 m/s
(c) 1×10-4 m/s
(d) 5×10-4 m/s
Question 4.
The ratio of the e/m values of an electron to that of a proton?
[Level: Moderate]
(a) 1833:1
(b) 1:1833
(c) 2:1
(d) 1:2
Question 5.
Photoelectric emission is observed from a surface for frequencies v1 and v2
of incident radiations (
). If the maximum kinetic energy of photoelectrons in the two cases are in the ratio 3:1, then the threshold frequency v0 is given by:
[Level: Difficult]
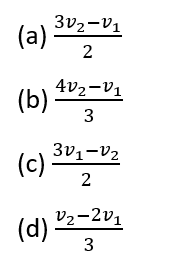
Question 6.
Which of the following transitions in a hydrogen atom corresponds to the Balmer series?
[Level: Easy]
(a) n=5 to n=3
(b) n=3 to n=2
(c) n=4 to n=1
(d) n=3 to n=1
Question 7.
If the speed of the electron in the third Bohr orbit of a hydrogen atom is z, then the speed of the electron in the sixth Bohr orbit is:
[Level: Moderate]
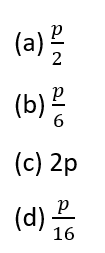
Question 8.
The atomic number of manganese is 26. What will be the magnetic property of Fe3+ ?
[Level: Difficult]
(a) Diamagnetic
(b) Paramagnetic
(c) Ferromagnetic
(d) None of these
Question 9.
If the total energy of an electron in the 3rd shell of a hydrogen atom is −1.51 eV, then its potential energy in the 1st excited state would be:
[Level: Difficult]
(a) −3.02 eV
(b) +3.02 eV
(c) +1.51 eV
(d) −1.51 eV
Question 10.
Identify the incorrect set of quantum numbers.
[Level: Moderate]
(a) n=5, l=4, m=−4, s=+1/2
(b) n=3, l=2, m=1, s=−1/2
(c) n=4, l=3, m=3, s=+1/2
(d) n=3, l=1, m=2, s=+1/2
Question 11.
The total energy in the third orbit of the hydrogen atom is given by:
[Level: Difficult]
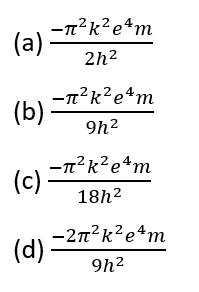
Question 12.
Which of the following statements is never true for cathode rays?
[Level: Easy]
(a) They possess kinetic energy
(b) They are electromagnetic waves
(c) They produce heat
(d) They produce mechanical pressure
Question 13.
Which of the following electronic configurations violates the Aufbau principle? [Level: Moderate]
(a) 1s² 2s² 2p⁶ 3s²
(b) 1s² 2s² 2p⁶ 3p¹
(c) 1s² 2s² 3s² 3p³
(d) 1s² 2s² 2p⁶ 3d¹
Question 14.
The electronic configuration of an element is 1s2 ,2s2 ,2p6 ,3s2 ,3p6 ,4s2 ,3d9 . This represents its:
[Level: Moderate]
(a) excited state
(b) ground state
(c) cationic form
(d) anionic form
Question 15.
The angular momentum of an electron in a circular orbit of radius r in a hydrogen atom is proportional to:
[Level: Easy]
(a) r
(b) r2
(c)
(d) 1/r
Question 16.
The number of spherical nodes in 3p orbitals are:
[Level: Easy]
(a) none
(b) one
(c) two
(d) three
Question 17.
For a d-electron, the orbital angular momentum is:
[Level: Moderate]
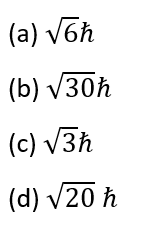
Question 18.
Which of the following species has 4 lone pair of electrons?
[Level: Moderate]
(a) I
(b) O-
(c) Cl-
(d) He
Question 19.
Which pair of ions has an identical electronic configuration?
[Level: Moderate]
(a) Cr3+ , V3+
(b) Ni2+ , Fe2+
(c) Co2+,Fe3+
(d) Cu+ , Zn2+
Question 20.
Identify the orbital that has 1 angular node and 3 total nodes.
[Level: Moderate]
(a) 5d
(b) 5p
(c) 4p
(d) 3d
**********
In summary, problem-solving after learning a theoretical concept on CBSE Structure of Atom NEET Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on NEET Chemistry Structure of Atom NCERT Chapter 2
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on NEET Chemistry Structure of Atom NCERT Chapter 2 useful.
Last but not least, to get the best hold on NEET Chemistry Structure of Atom NCERT Chapter 2, do not forget to check out: